Our lab is a neuroimmunology lab, primarily investigating mechanisms of the interplay between the immune system and central nervous system in health and disease. Ongoing projects focus on leukodystrophies, macrophages and pericyte interactions, and the immunomodulatory effects of umbilical cord blood.
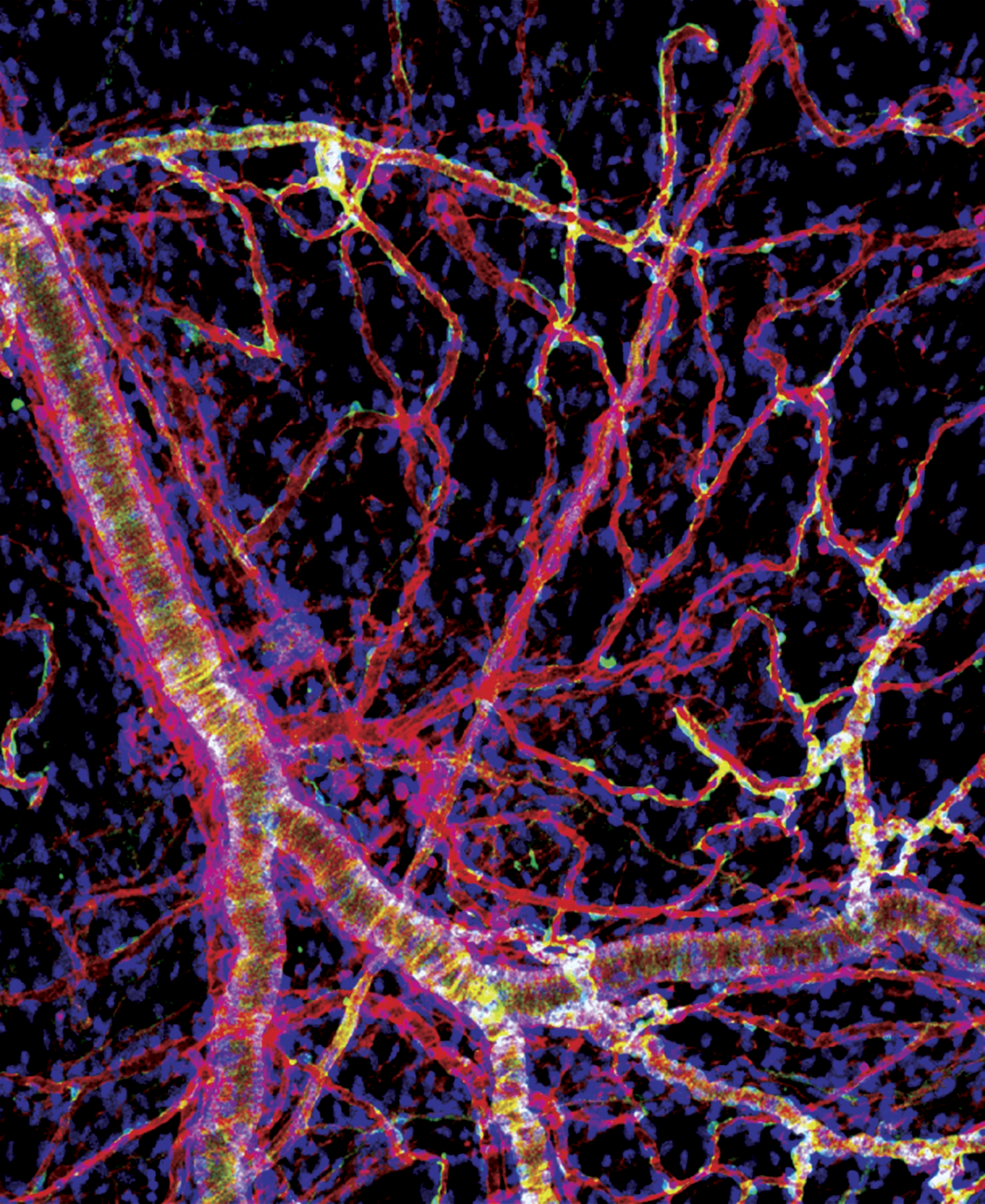
Multiple Sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) where infiltrating T cells ultimately lead to the destruction of myelin. T cells initially accumulate in the perivascular space of the brain and the meninges where they must interact with antigen presenting cells prior to activating and infiltrating into the parenchyma. This suggests immune interactions in the perivascular space may serve as a checkpoint and determine the fate of infiltrating T cells. Macrophages and pericytes are two important cells in the perivascular space of the brain and meninges. Under homeostatic conditions, border macrophages have an immuno-surveillant phenotype and express surface markers typical for macrophages that help with healing tissues. Pericytes also reside in the perivascular space and are a key component of the neurovascular unit where they regulate blood flow, angiogenesis, the blood-brain barrier (BBB), and neuroinflammation. Although each cell type has been implicated in multiple sclerosis (MS), the communication between the 2 cell types has not been described. Our preliminary data demonstrate that cultured pericytes suppress the activation of T cells through engaging macrophages, reprogramming them to downregulate genes need for antigen presentation and T cells activation. Pericytes/macrophage interactions are mediated by lipoprotein receptor-related proteins (LRP) on macrophages and dependent on p-bodies (membrane-less, cytoplasmic organelles that contain mRNAs enriched in regulatory functions) in pericytes. When we deleted pericytes in vivo, CNS antigen specific T cells infiltrated the perivascular space of the meninges in a manner that was dependent on macrophages, and further infiltrate into the parenchyma when triggered by a second signal from the parenchyma. We hypothesize that under homeostatic conditions, pericytes communicate with perivascular and meningeal macrophages to maintain them in an immunosuppressive and surveillant state, but in MS, communication between pericytes and macrophages breaks down, sending CNS macrophages into a proinflammatory state that contributes to T cell activation and infiltration into the brain. We are working to understand if pericytes instruct perivascular macrophages to inhibit brain-specific T cells from entering the parenchyma, investigate whether pericytes reprogram CNS macrophages in vivo, and determine if macrophages must engulf components of pericytes in order to be reprogramed to suppress T cells.
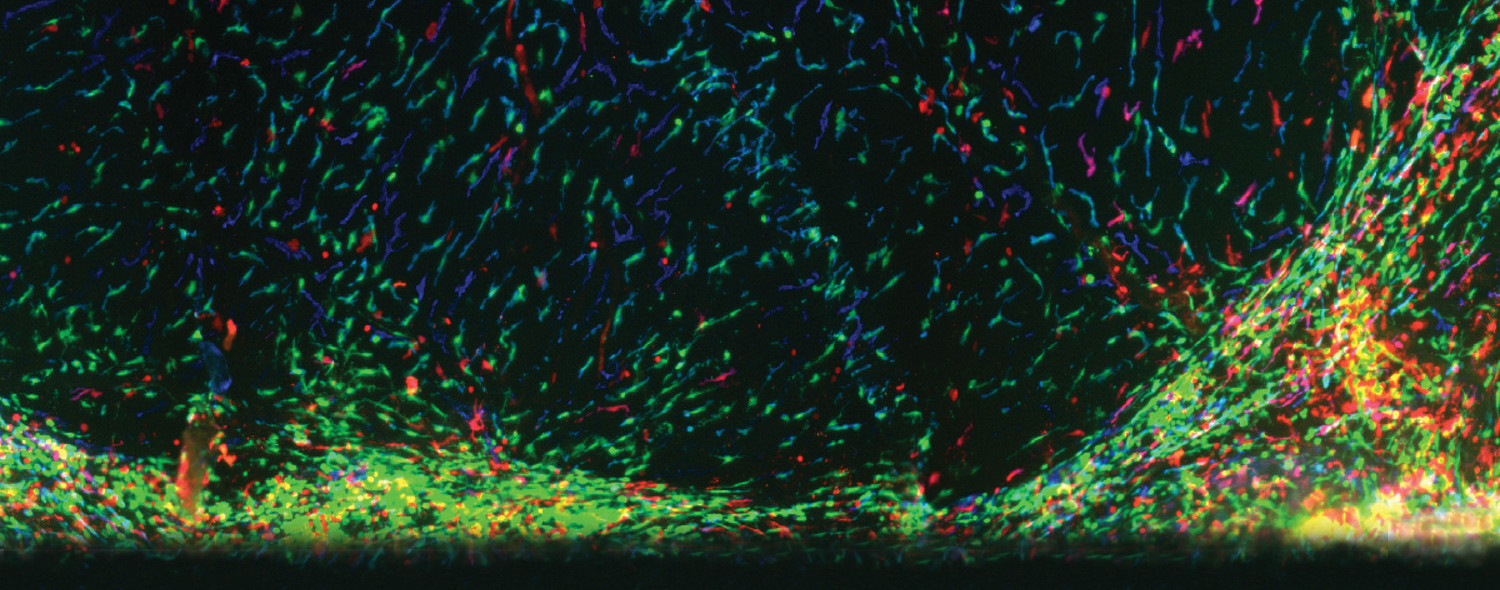
Patients with Krabbe disease have a deficiency in galactosylceramidase (GALC), caused by mutations, that lead to a toxic buildup of the sphingolipid psychosine in cells of the nervous system. The only approved disease-modifying therapy is hematopoietic stem cell transplants (HSCTs), that must be performed in presymptomatic patients to be beneficial. Presymptomatic HSCT in patients with Krabbe can slow the progression of pathology in the central nervous system (CNS), but the disease continues to progress as a chronic peripheral neuropathy. Why the peripheral nervous system (PNS) is less mendable by HSCT is unknown. This question remains a critical barrier for these patients diagnosed with Krabbe disease to lead a healthy life.
Our lab is studying a group of devastating and fatal childhood diseases called leukodystrophies. Leukodystrophies are inherited disorders affecting the myelin, the protective covering of nerve fibers in the brain and spinal cord. Symptoms often include developmental delays, loss of previously acquired skills, muscle stiffness (spasticity), coordination problems, difficulty walking, and behavioral changes. As the disease progresses, affected individuals may also experience seizures, vision and hearing loss, and cognitive decline. Most leukodystrophies are caused by genetic mutations that break a critical component in all cells. So, a common strategy is to replace these components. The problem is, even with state-of-the-art techniques, like gene therapy, these strategies fail because it’s impossible to hit all cells and usually the timing of treatment is too late. We are taking a different approach and instead of looking at individual cells, we are studying the immune environment and the fluid (called the cerebrospinal fluid or CSF) that bathes nerve cells. We are measuring and manipulating immune cells associated with nervous tissues and the CSF to understand the disease and are developing novel cell therapies to promote myelin repair.
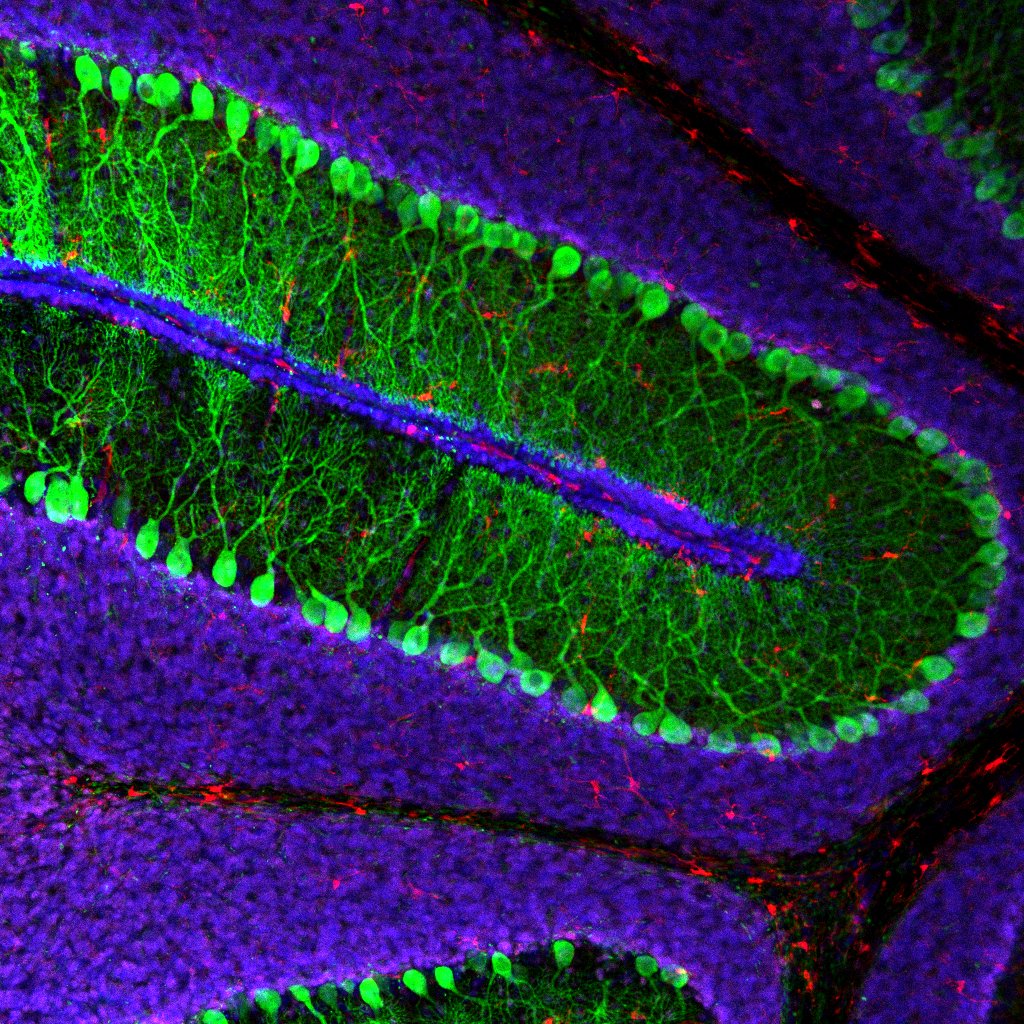
T cells support proper brain function and mice lacking T cells have behavioral deficits, such as decreased learning and memory and social withdrawal. Further, alterations in T cell function have been described in many neurological disorders. Interestingly, T cells are not present in the parenchyma of a healthy brain; yet they patrol the meninges and likely influence neuronal activity via the release of soluble cytokines. We are investigating how cytokines, and other immune-cell derived molecules, can regulate neural circuits and how an imbalance in these molecules might affect the brain in autism spectrum and other related disorders.
Cord blood and birthing tissues contains a rich source of immune-modulating components including, stem cells, immune cells, and soluble factors. Recent work has demonstrated the efficacy of using cord-blood derived components to promote myelination and increase function in neurological conditions such as multiple sclerosis, autism spectrum disorder, and aging. We are investigating how cord-blood derived cells and products can be used to promote healthy brain function and alleviate behavioral dysfunction in these devastating neurological conditions.
